Usual Dose & Administration
Usual Adult Dose
600 mg q12h
Adjustment of Dose & Administration
Indication-Specific Adjustment
Non-TB Mycobacterium infection: 600 mg daily
Renal Adjustment
No adjustment in renal dysfunction
If applicable, administer dose after hemodialysis
Drug-Specific Information
IV to PO Conversion:
600 mg IV q12h = 600 mg PO q12h
Bioavailability: 100%
Toxicities
- Thrombocytopenia, neutropenia (more common with treatment > 10-14 days)
- Serotonin syndrome in patients receiving serotonergic agents
- Linezolid-induced serotonin syndrome is rare. Use is generally safe in patients on only one serotonergic agent; however, risk may be increased with select agents, multiple concurrent serotonergic agents, and/or higher doses. See here for further information.
- Optic and peripheral neuritis (more common with long term treatment)
- Lactic acidosis
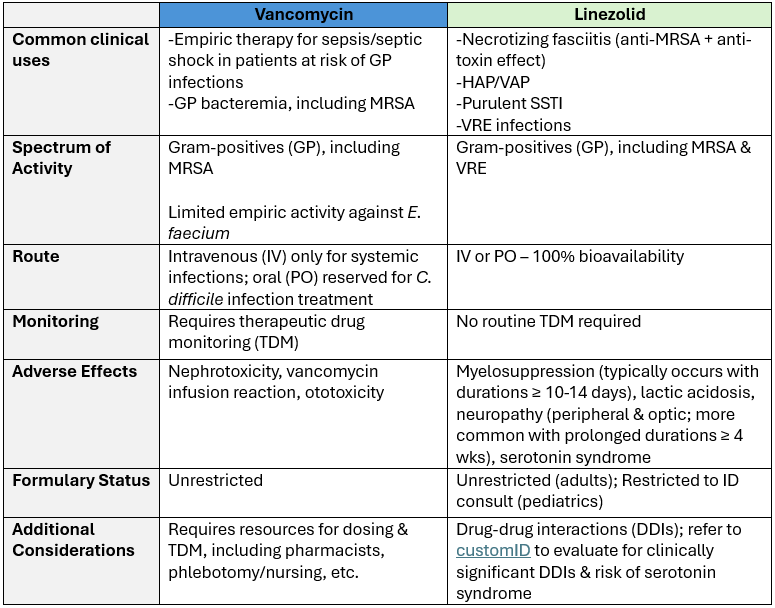
Table. Comparing anti-MRSA therapy (vancomycin and linezolid)
Restricted Use
Linezolid is unrestricted for adults as of March 2025
General Notes
- Up-to-date cost information, click here
- IV antimicrobials outpatient (OPAT) dosing, click here
- Obesity dosing weight recommendations here
- Helpful drug-drug interaction check website here
- When dosing guidance is provided it is important to note the following:
Fixed (ie non weight-based) doses in adults are historically based on a 70 kg patient. Specific disease states or individual patients may warrant dosages that differ from the above recommendations. Since product-specific criteria for dose adjustment based on creatinine clearance exist, consult product information regarding specific recommendations for dosage adjustment based on estimated creatinine clearance.